All published articles of this journal are available on ScienceDirect.
Characteristics and Prognostic Value of the RS2070744 Polymorphism in the NOS3 Gene in Patients with Heart Failure with Reduced Ejection Fraction
Abstract
Introduction
Heart failure with reduced ejection fraction (HFrEF) is a severe clinical condition associated with high mortality and morbidity rates. The –786T/C (rs2070744) polymorphism in the NOS3 gene may influence nitric oxide production and alter cardiovascular outcomes in HFrEF patients.
Methods
A cross-sectional descriptive study was conducted at Bac Lieu General Hospital, Vietnam from April 2023 to June 2024. Ninety patients with HFrEF (LVEF <40%) underwent genotyping for NOS3 rs2070744 polymorphism using RFLP-PCR. Patients were followed up at 3 and 6 months for heart failure-related readmission and mortality outcomes.
Results
Of the 90 patients (mean age 64.5 ± 13.3 years; 53.3% male), genotype frequencies were TT (61.1%), CT (32.2%), and CC (6.7%). At 3 months, 32.2% were readmitted for heart failure and 10.0% died; at 6 months, readmission and mortality rates were 25.9% and 8.6%, respectively. The CC genotype was associated with significantly higher readmission and mortality rates at 3 months (p<0.05). Multivariate Cox regression identified CC/CT genotype (HR=4.24; 95% CI=1.03–17.39; p<0.05) and dyslipidemia (HR=8.63; 95% CI=2.12–41.03; p<0.05) as independent predictors of mortality.
Discussion
The presence of the C allele may influence adverse cardiovascular outcomes in HFrEF patients, potentially via modulation of nitric oxide production, endothelial dysfunction, and autonomic dysregulation.
Conclusion
The rs2070744 polymorphism in the NOS3 gene may serve as a prognostic biomarker for poor outcomes in HFrEF. Patients with CC/CT genotypes and dyslipidemia have higher risks of readmission and mortality.
1. INTRODUCTION
Heart failure is a severe condition with a high mortality rate, representing the final pathway for most cardiovascular diseases [1]. It adversely impacts the quality of life and significantly contributes to the global healthcare cost [2]. The prevalence of heart failure varies significantly with age across different countries and regions. In 2017, the highest prevalence rates were recorded in Central Europe, North Africa, and the Middle East, ranging from 1,133 to 1,196 per 100,000 population, while lower rates were recorded in Eastern Europe and Southeast Asia, ranging from 498 to 595 per 100,000 population [3, 4]. Advancements in diagnostic and therapeutic approaches have led to the emergence of numerous studies that have significantly enhanced treatment efficacy for this patient population. Notably, the use of spironolactone and sodium-glucose cotransporter-2 inhibitors (SGLT2i) has been reported to improve symptoms, cardiac function, and prognosis in patients with heart failure with preserved ejection fraction [5, 6]. Heart failure with reduced ejection fraction (HFrEF) represents a severe clinical subtype, characterized by a significant decline in left ventricular systolic function, with a left ventricular ejection fraction (LVEF) of less than 40% [7]. Patients with heart failure with reduced ejection fraction (HFrEF) continue to experience a substantial burden of adverse outcomes over both the short and long term, and frequently exhibit increased insulin resistance as a comorbidity [8, 9]. In South Korea, an analysis of 1,527 patients with HFrEF reported a mortality rate of 3.8% at 60 days and 9.2% at one year, while heart failure-related readmission rates were 3.1% at 60 days and 9.8% at one year [10]. Similarly, the JCARE-CARD study in Japan documented a one-year mortality rate of 8.9% [11]. A meta-analysis conducted by Agustín Ciapponi and colleagues reported heart failure-related readmission rates of 33%, 28%, 31%, and 35% at 3, 6, 12, and 24-60 months, respectively. Additionally, the overall one-year mortality rate was 24.5%, with a higher rate observed among patients with heart failure with reduced ejection fraction (HFrEF) [12]. This suggests that, in addition to traditional risk factors, there remains a gap in understanding the pathogenesis of this disease group. Data indicates that factors influencing myocardial metabolism may contribute to the progression of chronic heart failure [13]. Myo-inositol, a metabolite transported by the sodium-myo-inositol co-transporter 1 (SMIT-1), has been found at high concentrations to induce oxidative stress, thereby impairing cardiac function and promoting adverse cardiac remodeling [14]. Jing Ye et al. reported that elevated plasma levels of Interleukin-11 significantly increased the occurrence of cardiovascular events [15]. Similarly, Kielin/Chordin-like protein (KCP), a secreted protein that regulates the expression of transforming growth factor-beta (TGF-β) and bone morphogenetic proteins (BMPs), has been identified as being associated with structural cardiac changes in the progression of heart failure [16].
Recently, nitric oxide (NO) has been shown to be a small lipophilic molecule that plays a crucial role in cardiovascular regulation. One of its most important functions is controlling vascular tone by activating soluble guanylate cyclase (sGC), a heme-containing heterodimeric enzyme expressed in vascular smooth muscle cells. In response to NO, sGC activity increases approximately 200-fold, leading to the rapid conversion of guanosine triphosphate (GTP) into cyclic guanosine monophosphate (cGMP). This, in turn, activates protein kinase G-1 (PKG-1), resulting in a reduction of intracellular free calcium levels and subsequent vasodilation [17]. The regulation of NO production is primarily controlled by nitric oxide synthase 3 (NOS3), an enzyme encoded by the NOS3 gene. This gene is located on chromosome 7q35-7q36 in humans, spanning approximately 21–22 kb and comprising 25 introns and 26 exons. Consequently, any abnormalities in the NOS3 gene may affect NO production capacity [17]. The rs2070744 polymorphism, also known as –786T/C, is in the promoter region of the NOS3 gene. This polymorphism involves the substitution of thymidine with cytosine, leading to reduced gene expression and decreased nitric oxide levels in the serum. It is a critical factor in the pathogenesis and prognosis of heart failure patients [18]. The impact of this polymorphism on vascular tone includes coronary vasoconstriction and an increased risk of myocardial infarction onset. Additionally, it disrupts the cardiac autonomic nervous system, elevating the risk of sudden cardiac death and accelerating the progression of heart failure [19-21]. However, data on the prognostic significance of this polymorphism remain limited. Therefore, we conducted this study to identify the characteristics and prognostic value of the rs2070744 polymorphism in patients with heart failure with reduced ejection fraction (HFrEF).
2. METHODS
2.1. Study Design and Population
This study was a cross-sectional descriptive analysis, utilizing convenience sampling to include all patients with heart failure with reduced ejection fraction (HFrEF) who were hospitalized in the Cardiology Department of Bac Lieu General Hospital between April 2023 and June 2024. Inclusion and exclusion criteria were established to select appropriate participants for the study. Patients were diagnosed with HFrEF according to the 2021 ESC guidelines, meeting the following criteria: (1) clinical manifestations of heart failure, (2) elevated NT-proBNP levels ≥125 pg/mL, and (3) LVEF less than 40% on echocardiography [7]. Exclusion criteria included: (1) chronic kidney disease with an estimated glomerular filtration rate (eGFR) <30 mL/min/1.73m2, (2) hyperkalemia >5 mmol/L, (3) active cancer, (4) psychiatric disorders or dementia impairing the ability to respond to questions, and (5) loss to follow-up. After the follow-up period, a total of 90 HFrEF patients meeting the criteria were included in the study for analysis.
2.2. Data Collection
All patients were assessed for demographic characteristics, including age, gender, and body mass index (BMI), with overweight and obesity defined as BMI ≥23 kg/m2 for Asian populations [22]. Waist circumference was measured using a measuring tape at the level of the anterior superior iliac spine, with abdominal obesity defined as >90 cm in men and >80 cm in women for Asian populations [23]. A clinical examination was conducted by a specialist physician immediately thereafter to document clinical symptoms of heart failure, blood pressure levels, and the NYHA classification at the time of admission [24] to assess the severity of heart failure. Comorbid conditions, such as hypertension, type 2 diabetes, dyslipidemia, and coronary artery disease, were also documented, and smoking status was defined according to the criteria set in the COMMIT (Community Intervention Trial) study [25].
2.3. Sample Sizes
We calculated the sample size using the proportion estimation formula, with α = 5%, d = 0.07, and p = 11.0%, which represents the prevalence of the CC genotype in the study by Terzi S et al. on the impact of rs2070744 polymorphism in the NOS3 gene on long-term mortality in patients with chronic heart failure [26]. The minimum required sample size was determined to be 77 patients; however, our study included a total of 90 patients. The sample size was calculated using the standard proportion formula (Eq. 1):
 |
(1) |
2.4. Genotyping
Previous studies have reported a thymine-to-cytosine point mutation at position –786T/C (rs2070744) in the NOS3 gene, which may alter its expression levels and enzymatic activity [19-21]. Based on this, to characterize this polymorphism in patients with heart failure, we conducted the following procedure: Two milliliters of blood were drawn from each patient into an EDTA anticoagulant tube, transported to the laboratory within 24 hours, and stored at -20°C until use. The NOS3 gene sequencing to detect rs2070744 polymorphism was performed using the RFLP-PCR technique at the Molecular Biology Laboratory, Can Tho University of Medicine and Pharmacy. The DNA extraction procedure involved using 200 µl of whole blood from each sample, which was processed with the QIAamp DNA Blood Mini Kit, including three main steps: (1) cell and nuclear membrane lysis to release DNA; (2) washing away proteins and impurities; and (3) DNA collection. To ensure the quality of the extracted DNA, the concentration was measured, and its purity was assessed using a spectrophotometer, with the following parameters: concentration >10 ng/µl; purity OD260/280 = 1.8 – 2.0.
The RFLP-PCR technique, with the PCR amplification process, was performed using the following primers:
Forward primer: 5'- TGG AGA GTG CTG GTG TAC CCC A -3'
Reverse primer: 5'- GCC TCC ACC CCC ACC CTG TC -3'
Each PCR reaction was performed with a final volume of 25 µl, consisting of 3 µl of DNA template, 4 µl of primers (2 µl for each forward and reverse primer), 0.5 µl of Taq DNA polymerase (Gen Fanavaran, Iran), 12.5 µl of Taq DNA Polymerase Master Mix Red (Ampliqon, Denmark), and 5 µl of double-distilled water (nuclease-free). The thermal cycling process consisted of an initial denaturation at 94°C for 2 minutes, followed by 35 cycles of denaturation at 94°C for 1 minute, annealing at 62°C for 1 minute, and extension at 72°C for 1 minute. The amplification process concluded with a final extension at 72°C for 7 minutes. After the thermal cycling process, the PCR products were digested with restriction enzymes according to the manufacturer's instructions. The restriction enzyme-digested products were then analyzed by electrophoresis on a 2% agarose gel, and the results were recorded and analyzed.
2.5. Follow-up Outcomes
All patients with HFrEF were treated according to the ESC guidelines, including medications that improve heart failure symptoms and prognosis, such as agents targeting the renin-angiotensin-aldosterone system (ARNI/ACEi/ARB), beta-blockers, mineralocorticoid receptor antagonists, and sodium-glucose cotransporter-2 inhibitors (SGLT2i) [7]. After discharge, patients were followed up for 3 months and 6 months. Survival status and clinical events were assessed by contacting patients and their family members via telephone, with verification through the review of hospital medical records. The clinical events monitored included readmission due to heart failure and mortality from all causes. According to the 2021 ESC diagnostic criteria, heart failure rehospitalization is defined as a hospital readmission following discharge due to acute clinical manifestations of heart failure, including acute dyspnea and fluid overload, requiring intravenous therapeutic intervention [7]. Mortality included both cardiovascular and all-cause death, recorded based on medical records. At the end of the follow-up period, data was compiled and statistically analyzed.
2.6. Statistical Analysis
Data was analyzed using SPSS 20.0 software. Qualitative variables were presented as frequencies (percentages), while mean (± standard deviation, SD) was used for normally distributed quantitative variables, and median (interquartile range, IQR) for non-normally distributed quantitative variables. The normality of the distribution was assessed using the Kolmogorov-Smirnov test, and it was considered normal if the significance level (Sig.) was greater than 0.05. The differences between qualitative variables were described using the Chi-square test. The prognostic ability of the genotype characteristic, rs2070744 polymorphism allele, was assessed based on Kaplan-Meier survival curves. Finally, univariate and multivariate Cox regression analyses were conducted to identify independent prognostic factors for patients with reduced ejection fraction failure.
2.7. Ethical Approval
All patients were informed about the study objectives and methods, and provided written consent before participation. They were also allowed to withdraw from the study at any stage. All information collected was used solely for research purposes and will not be published without the patient's consent. The costs for genetic sequencing and any other expenses arising during the study were fully covered by the research team. The study was approved by the Biomedical Research Ethics Committee of the Can Tho University of Medicine and Pharmacy and adhered to the ethical principles outlined in the Helsinki Declaration.
3. RESULTS
The demographic and clinical characteristics of the study population are presented in (Table 1). Among the 90 patients with reduced ejection fraction heart failure, the mean age was 64.5 ± 13.3 years, with 53.3% being male. Most patients were hospitalized with NYHA III, accounting for 74.4%. The most common comorbidities were coronary artery disease (96.7%) and dyslipidemia (95.6%). The average EF value recorded was 32.5 ± 6.7%.
| Characteristics | Total (n=90) | |
|---|---|---|
| General Characteristics | ||
| Age (years) | 64.5 ± 13.3 | |
| Male (%) | 48 (53.3) | |
| Obesity (%) | 20 (22.3) | |
| Waist circumference (cm) | 83.9 ± 6.9 | |
| Systolic blood pressure (mmHg) | 127.8 ± 21.6 | |
| Diastolic blood pressure (mmHg) | 77.9 ± 10.0 | |
| Clinical Characteristics | ||
| NYHA | Class II | 09 (10.0) |
| Class III | 67 (74.4) | |
| Class IV | 14 (15.6) | |
| Hypertension (%) | 73 (81.1) | |
| Type 2 Diabetes Mellitus (%) | 31 (34.4) | |
| Dyslipidemia (%) | 86 (95.6) | |
| Coronary Artery Disease (%) | 87 (96.7) | |
| Smoking (%) | 30 (33.3) | |
| Average EF (%) | 32.5 ± 6.7 | |
The characteristics of the rs2070744 polymorphism in the NOS3 gene are presented in (Table 2). The most common genotype is TT, observed in 61.1%, followed by CT and CC at 32.2% and 6.7%, respectively. In terms of allele frequency, the T allele is present at 77.2%, and the C allele is present at 22.8%.
When analyzing the clinical characteristics based on the rs2070744 polymorphism of the NOS3 gene, it was found that the proportion of patients with the CC/CT genotypes suffering from type 2 diabetes mellitus was higher compared to those with the TT genotype, at 48.6% versus 25.5% (p<0.05). No significant differences were found between the two genotype groups regarding gender, comorbidities such as hypertension, dyslipidemia, coronary artery disease, and the average EF values (Table 3).
| Characteristics | Total (n=90) |
|---|---|
| Genotype (n=90) | |
| CC | 06 (6.7) |
| CT | 29 (32.2) |
| TT | 55 (61.1) |
| Allele (n=90x2) | |
| C | 41 (22.8) |
| T | 139 (77.2) |
| Characteristics |
CC/CT Carriers (n=35) |
TT Carriers (n=55) |
p | |
|---|---|---|---|---|
| Gender | Male | 22 (62.9) | 26 (47.3) | 0.149 |
| Female | 13 (37.1) | 29 (52.7) | ||
| Hypertension | 29 (82.9) | 44 (80.0) | 0.736 | |
| Type 2 Diabetes mellitus | 17 (48.6) | 14 (25.5) | 0.024 | |
| Dyslipidemia | 34 (97.1) | 52 (94.5) | 1.0 | |
| Coronary Artery Disease | 34 (97.1) | 53 (96.4) | 1.0 | |
| EF (%) | 31.9 ± 7.1 | 32.9 ± 6.4 | 0.485 | |
At the 3-month follow-up, 29 patients were readmitted due to heart failure (32.2%), with 37.9% of them carrying the CC/CT genotype. Similarly, 9 patients died (10.0%), of which 66.7% had the CC/CT genotype. At the 6-month follow-up, 21 patients were readmitted for heart failure (25.9%), and 7 patients died (8.6%). Among these, 33.3% and 42.9% of patients with the CC/CT genotype experienced readmission and death, respectively (Table 4).
| Events |
CC/CT Carriers (n=35) |
TT Carriers (n=55) |
p |
|---|---|---|---|
| After 3 months | |||
| Heart failure-related readmission | 11 (37.9) | 18 (62.1) | 0.898 |
| Death | 6 (66.7) | 3 (33.3) | 0.085 |
| After 6 months | |||
| Heart failure-related readmission | 7 (33.3) | 14 (66.7) | 0.784 |
| Death | 3 (42.9) | 4 (57.1) | 0.697 |
According to the Kaplan-Meier analysis (Fig. 1A), at the 3-month time point, patients with the CC genotype had a higher probability of heart failure-related readmission compared to the other two genotypes (p<0.05). However, at the 6-month time point (Fig. 1B), the difference was not statistically significant (p>0.05).
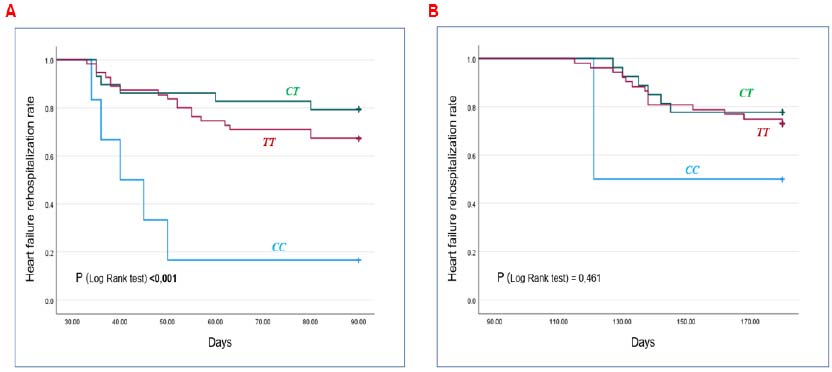
Kaplan-Meier curves estimating heart failure readmission events according to the genotype characteristics of the rs2070744 NOS3 gene.

Kaplan-Meier curves estimating mortality events based on the genotype characteristics of the rs2070744 NOS3 gene polymorphism.
Similarly, regarding mortality events, the Kaplan-Meier analysis shown in Fig. (2A) indicates that patients with the CC genotype had a lower cumulative survival rate at 3 months post-discharge compared to those with the CT and TT genotypes (p<0.001). However, at the 6-month time point (Fig. 2B), the difference in mortality between the groups was not statistically significant (p>0.05).
| Factors | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Genotype CC/CT | 3.44 (0.86-13.75) |
0.021 | 4.24 (1.03-17.39) |
0.045 |
| Age (years) | 0.97 (0.82-1.02) |
0.214 | - | - |
| Hypertension | 2.27 (1.07-3.03) |
0.055 | - | - |
| Type 2 Diabetes mellitus | 1.92 (0.40-9.24) |
0.042 | 2.02 (1.11-7.83) |
0.334 |
| Dyslipidemia | 7.20 (1.48-34.95) |
0.014 | 8.63 (2.12-41.03) |
0.012 |
| Coronary Artery Disease | 4.31 (2.12-13.47) |
0.033 | 5.26 (2.39-15.07) |
0.076 |
| Factors | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Genotype CC/CT | 1.35 (0.30-6.03) |
0.694 | - | - |
| Age (years) | 0.98 (0.93-1.04) |
0.612 | - | - |
| Hypertension | 5.62 (3.01-17.63) |
0.015 | 6.66 (2.72-21.68) |
0.031 |
| Type 2 Diabetes mellitus | 1.36 (0.31-6.08) |
0.686 | - | - |
| Dyslipidemia | 9.11 (3.12-12.12) |
0.027 | 10.09 (4.31-15.22) |
0.125 |
| Coronary Artery Disease | 3.33 (1.12-7.68) |
0.013 | 5.67 (2.21-9.63) |
0.224 |
The univariate Cox regression analysis (Table 5) found that the factors, including the CC/CT genotype, type 2 diabetes, dyslipidemia, and coronary artery disease, were associated with mortality events at the 3-month time point. However, in the multivariate Cox regression model, only the CC/CT (HR=4.24; 95% CI=1.03-17.39; p<0.05) and dyslipidemia (HR=8.63; 95% CI=2.12-41.03; p<0.05) were independent predictors of mortality.
At the 6-month time point (Table 6), univariate Cox regression identified hypertension, dyslipidemia, and coronary artery disease as factors associated with mortality. However, in the multivariate model, only hypertension remained an independent predictor of mortality, with a hazard ratio (HR) of 6.66 (95% CI = 2.72-21.68; p<0.05).
4. DISCUSSION
4.1. Principal’s Findings
Our study observed that 22.8% of patients with reduced ejection fraction heart failure carried the C allele. The CC genotype was associated with a more severe prognosis, with a higher probability of heart failure-related readmission and lower cumulative survival at 3 months post-discharge compared to the TT and CT genotypes. Regarding mortality events, after adjusting for risk factors, the CC/CT genotype and dyslipidemia were independent predictors of mortality at 3 months. However, at 6 months, only hypertension as a comorbid condition remained an independent prognostic factor.
4.2. Strengths and Weaknesses of the Study
Our study involved a clearly defined sample collection process with specific inclusion and exclusion criteria. All participants were enrolled voluntarily, and the gene sequencing was conducted in a modern molecular biology laboratory, ensuring reliable results with well-characterized genotypes and alleles of the rs2070744 NOS3 gene, which can be reproduced. The event follow-up period was relatively long, at 3 and 6 months, providing valuable information regarding long-term prognosis. Notably, the C allele was identified as a risk allele, with patients carrying the CC genotype having a more severe prognosis. The CC/CT genotype was an independent predictor of mortality.
| Author\Refs. | Sample Size | Population | Results |
|---|---|---|---|
| Terzi et al. [26] (2017) |
91 patients in the disease group 30 patients in the control group |
CHF patients | TT 54%. CT 35%. CC 11%. |
| Bielecka-Dabrowa et al. [27] (2016) |
HFpEF: 51 patients HFrEF + DCM: 27 patients HFrEF + ICM: 32 patients |
CHF patients | HFpEF: TT 49.0%, CT 51.0% HFrEF + DCM: TT 18.0%, CT 78.0%, CC 4.0% HFrEF + ICM: TT 53.0%, CT 47.0% |
| Bielecka-Dabrowa et al. [28] (2017) |
HFpEF và HFmrEF: 110 patients |
CHF patients | TT 43.0%, CT 56.0%, CC 1.0% |
| Abdullayeva et al. [18] (2014) | 81 male patients | CHF patients | TT 55.6%. CT 43.2%. CC 1.2%. |
However, our study was conducted in a single hospital or region with a limited sample size, which may lead to differences in certain demographic and clinical characteristics. Additionally, we focused on only two outcomes: readmission due to heart failure and mortality, thus not covering all aspects of prognosis. Therefore, further research with a larger sample size, conducted across multiple centers, considering treatment aspects and evaluating a broader range of outcomes, is necessary to fully describe the characteristics of rs2070744 and capture the full prognostic potential of this polymorphism.
4.3. Possible Explanations and Comparisons with other Studies
The study recorded a mean age of 64.5 ± 13.3 years, with most patients being male, accounting for 53.3% (Table 1). Regarding the rs2070744 polymorphism, in 2016, Agata Bielecka-Dabrowa reported the distribution in heart failure with preserved ejection fraction (HFpEF) patients as 49.0% for the TT genotype, 51.0% for CT, and no cases with the CC genotype. In contrast, patients with heart failure with reduced ejection fraction due to dilated cardiomyopathy had a genotype distribution of 18.0% TT, 78.0% CT, and 4.0% CC, while HFrEF patients with ischemic heart disease had TT and CT genotypes at 53.0% and 47.0%, respectively [27]. These results differ significantly from our study, where the TT genotype was the most common at 61.1%, followed by CT and CC at 32.2% and 6.7%, respectively (Table 2). In 2017, this author observed in patients with HFpEF and HFmrEF that the genotypic distribution was 43.0% TT, 56.0% CT, and 1.0% CC, with allele frequencies of 43.0% for T and 57.0% for C. Clinically, patients carrying the risk allele C exhibited lower EF, eGFR (MDRD), and both systolic and diastolic blood pressure, while having a higher NYHA class [28]. Similarly, Abdullayeva et al. assessed the rs2070744 polymorphism in Uzbekistani patients with chronic heart failure aged 41 to 70 years, finding a significant deviation from Hardy-Weinberg equilibrium, with an overrepresentation of heterozygotes. The genotypic distribution was 55.6% TT, 43.2% CT, and 1.2% CC [18]. Additionally, in a study of type 2 diabetes patients, M. Boronat found genotype frequencies of 28.5% TT, 41.1% CT, and 30.5% CC, with the risk allele C occurring in 51.0% of patients, compared to 49.0% for allele T [29]. Similarly, Andrzej Pawlik analyzed 246 patients with a history of coronary artery disease, comparing them with a control group. The genotype frequencies of CC and CT in the control group were 17.55% and 44.68%, respectively, while in the disease group, the frequencies were 13.93% and 43.03%. Regarding the disease-causing allele, the author found that the C allele appeared in the control group at a rate of 39.89%, compared to 35.45% in the disease group [19]. Sahar Gamil studied Sudanese individuals with hypertension, finding genotype frequencies of CC, CT, and TT at 6.6%, 46.1%, and 47.4%, respectively, compared to 6.1%, 28%, and 65.9% in the control group [30]. Although several preliminary studies have evaluated the rs2070744 polymorphism in heart failure patients (Table 7), there is still limited evidence regarding heart failure with reduced ejection fraction. Differences in race and geographic region have influenced genetic stability, leading to varying genotype and allele frequency distributions among different authors [18].
In terms of prognosis, we found that at the 3-month time point, patients with the CC genotype had a higher probability of heart failure-related readmission and a lower cumulative survival rate compared to those with the CT and TT genotypes (p<0.05) (Figs. 1 and 2). Furthermore, the CC/CT genotypes, along with dyslipidemia, were identified as independent predictors of mortality (Table 5). Currently, there is limited research on the prognostic value of the rs2070744 polymorphism in heart failure patients. Sait Terzi's preliminary study on the effect of this polymorphism on long-term mortality in patients with chronic heart failure found a significant increase in mortality among patients with the CC genotype compared to those with the TT genotype (HR=2.4; 95% CI=1.01-5.60; p=0.04). In particular, the Kaplan-Meier analysis showed that the CC genotype was associated with a worse long-term prognosis, with a lower survival rate compared to the TT genotype (10% vs. 39%) [26]. Previous studies have suggested that the rs2070744 polymorphism is likely to impact NOS3 gene expression and serum nitric oxide levels. The –786T/C substitution in the promoter region reduces its activity by approximately 50%, which may lead to decreased NOS3 mRNA expression and a reduction in serum nitric oxide levels. Evidence indicates that this effect is closely associated with the development of coronary artery disease, vasospastic angina, and myocardial infarction, which are among the leading causes of heart failure or exacerbate existing heart failure conditions [19-21]. In addition to its role in regulating vascular tone, this polymorphism is also associated with endothelial dysfunction, promoting the development of dilated cardiomyopathy [27, 31]. Furthermore, the thymidine-to-cytosine substitution at rs2070744 in the nitric oxide synthase gene leads to an imbalance in the autonomic nervous system within the heart, which manifests as heart rate variability. Philip F. Binkley suggests that this factor may allow the rs2070744 polymorphism to serve as a prognostic marker for patients at risk of sudden cardiac death and rapid progression of heart failure [29]. Regarding dyslipidemia, no studies have yet provided evidence on the impact of dyslipidemia on prognosis in patients with HFrEF that specifically involves the rs2070744 polymorphism of the NOS3 gene. The prognostic value of dyslipidemia in HFrEF patients has been reported in several clinical studies; however, these studies did not examine genetic polymorphisms. Solmaz Norouzi evaluated factors influencing mortality in HFrEF patients using a prognostic model based on the Bayesian method, identifying hyperlipidemia as a significant variable associated with HFrEF-related mortality, with a hazard ratio (HR) of 0.34 (95% CI: 0.13–0.64), and with non-HFrEF-related mortality, with an HR of 0.60 (95% CI: 0.37–0.90). This suggests that hyperlipidemia may be closely associated with reduced survival time in heart failure patients [32]. Additionally, statin therapy has been reported to be associated with a reduction in both short-term and long-term all-cause mortality in patients with severe heart failure [33]. Regarding the interaction between NOS3 and dyslipidemia, Andrii Sydorchuk et al. investigated the rs5443 polymorphism of the GNB3 gene and the rs2070744 polymorphism of the NOS3 gene in patients with hypertension. Their findings indicated that rs5443, rather than rs2070744, was the polymorphism associated with hyperlipidemia in hypertensive patients [34]. These findings highlight the existing knowledge gap regarding the interaction between the rs2070744 polymorphism of NOS3 and dyslipidemia in predicting cardiovascular events in HFrEF patients. Further studies are needed to clarify this relationship in future research.
CONCLUSION
The study observed a relatively high prevalence of heart failure patients with reduced ejection fraction carrying genotypes containing the risk allele C, with the CC/CT genotype being 38.9%. Patients with the CC genotype had a more severe prognosis, characterized by a higher likelihood of heart failure-related readmission and a lower cumulative survival rate. The CC/CT genotype and dyslipidemia are independent determinants of mortality, highlighting their potential prognostic significance in patients with HFrEF.
AUTHORS' CONTRIBUTIONS
The authors confirm their contribution to the paper as follows: T.N.N.P., T.T.N.N., V.A.T.: Conceptualization; T.N.N.P., V.A.T.: Methodology; N.N.P., T.K.O.N.: Software; M.H.P., T.K.O.N., T.C.H.: Formal analysis; T.T.N.N.: Data curation: T.N.N.P., T.K.O.N.: Writing original draft preparation; T.N.N.P., T.T.N.N., M.H.P., T.K.O.N., T.C.H., V.A.T.: Writing reviews and editing.
LIST OF ABBREVIATIONS
| HFrEF | = Heart failure with reduced ejection fraction |
| HFpEF | = Heart failure with preserved ejection fraction |
| SGLT2i | = Sodium-glucose cotransporter-2 inhibitors |
| NO | = Nitric oxide |
| LVEF | = left ventricular ejection fraction |
| SMIT-1 | = Sodium-myoinositol co-transporter 1 |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Ethics Committee in Biomedical Research of Can Tho University of Medicine and Pharmacy (Approval No. 166, dated 16/03/2022).
HUMAN AND ANIMAL RIGHTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or research committee and with the 1975 Declaration of Helsinki, as revised in 2013.
CONSENT FOR PUBLICATION
All patients were informed about the study objectives and methods, and provided written consent before participation.
AVAILABILITY OF DATA AND MATERIAL
The data supporting the findings of the article are available upon reasonable request by contacting the corresponding author.
ACKNOWLEDGEMENTS
The authors would like to thank Can Tho University of Medicine and Pharmacy for creating favorable conditions for this study to be carried out.


