All published articles of this journal are available on ScienceDirect.
Paraparesis as the First Manifestation of Myocarditis Due to Cardiac Embolization into the Aorta Mimicking Guillain-Barré Syndrome: A Case Report
Abstract
Background
Guillain-Barré syndrome (GBS) is the most common cause of acute flaccid paralysis, which is characterized by ascending, rapid-onset, symmetrical limb weakness and sensory disturbances that are typically established 3-6 weeks after an antecedent infection, usually an upper respiratory tract infection.
Case Report/Case Representation
We present the case of a 19-year-old man who was acutely admitted to the emergency room due to the sudden onset and gradual worsening of symmetrical lower extremity paresis, sensory disturbance, and pain occurring 3 weeks after an upper respiratory tract infection.GBS was the initial diagnosis. However, this was ruled out after thorough neurological examination due to the rapid progression of neurological deficiency, intense pain, and the absence of a pulse in the lower extremity arteries. CT angiography revealed occlusion of the abdominal aorta, and an intracardiac thrombus was detected. The myocarditis complicated by intracardiac thrombus formation and subsequent embolization into the aorta was finally concluded as the actual reason for the patients' complaints, initially mimicking GBS.
Conclusion
This case report highlights a rare combination of two distinct, life-threatening conditions that together mimicked Guillain-Barré syndrome. The initial physical examination played a crucial role in establishing the correct differential diagnosis.
1. INTRODUCTION
Guillain-Barré syndrome (GBS), an acute inflammatory and usually demyelinating polyradiculoneuropathy, is the most common cause of acute flaccid paralysis worldwide [1, 2]. The annual global incidence of GBS is approximately 1-2/100,000 [3]. Typical clinical features of GBS include progressive and symmetric muscle weakness and absent or depressed deep tendon reflexes. The sensory symptoms (numbness, tingling, and pain) and dysautonomia may also be present. Typically, both sides of the body are involved. The patient typically presents within a few days to a week after the onset of symptoms. GBS symptoms typically progress over a period of two weeks. By four weeks after onset, > 90% of patients have reached the nadir of the disease. If the nadir is reached within 24 h or after four weeks of symptom onset, alternative diagnoses must be considered. Most patients present with an antecedent illness, most commonly upper respiratory tract infection, 3-6 weeks before the onset of progressive motor weakness [1, 2]. Polyradiculoneuropathy in Guillain-Barré syndrome (GBS) is caused by an autoantibody-mediated immune response, triggered by molecular mimicry between structural components of peripheral nerves and microbial antigens [1, 2].
Table 1 shows a comprehensive list of conditions causing acute flaccid paralysis that may mimic the neurological presentation of GBS. Table 1 can therefore be used in the differential diagnosis of GBS and at the same time for alternative explanations for GBS-like symptoms.
In this case report, we describe a differential diagnostic approach in a 19-year-old male who presented with acute paraparesis after an antecedent upper respiratory tract infection. The patient was initially suspected to have Guillain-Barré syndrome; further evaluation revealed an acute aortic occlusion caused by cardiac embolization due to intracardiac thrombus formation secondary to myocarditis. This case report highlights key roles in differential diagnosis and final decision-making and reviews how to manage these two life-threatening conditions.
2. CASE PRESENTATION
2.1. Chief Complaints
A 19-year-old Caucasian male was admitted to a local hospital via an ambulance after an acute-onset and gradually worsening symmetrical lower limb paresis, sensory disturbance, and pain.
2.2. History of Present Illness
Three weeks before emergency admission, his general practitioner prescribed antibiotics due to low-grade fever and symptoms of an upper respiratory tract infection, including cough, malaise, weakness, and chills. Clarithromycin at a dose of 500 mg once a day orally and cefixime at a dose of 400 mg once a day orally were administered. The fever persisted for a total of seven days during outpatient care.
2.4. Personal and Family History
He denied any family history of malignant, cardiovascular, pulmonary, inflammatory, or endocrinological diseases.
2.5. Physical Examination upon Admission
Upon physical examination, the patient seemed anxious and stressed with intense pain in the lower extremities. Loss of sensory function on the foot and foreleg bilaterally and moderate loss of motor function bilaterally, i.e., paraparesis, were also noted. The skin of the lower extremities was pale. There were no palpable pulsations in any artery of the lower extremities.
His body temperature was 36.7 °C. His hemoglobin saturation was 95%, and his blood pressure was 105/67 mmHg. The patient's heartbeat was regular, with a frequency of 98 beats/min.
Auscultation of the lungs revealed barely audible inspiratory crackles over the basal parts of the lungs.
2.6. Laboratory Examinations
The initial laboratory examination revealed elevated levels of leukocytes, creatinine, C-reactive protein, and N-terminal pro–B-type natriuretic peptide. All laboratory findings upon admission are listed in Table 2.
| Viruses Targeting Anterior Horn Cells or Motor Neurons | Acute Peripheral Neuropathies |
| • Poliomyelitis, non-polio enterovirus (enterovirus 71), West Nile virus | • Infections (e.g., herpes simplex virus, HIV) |
| • Herpes simplex virus, cytomegalovirus, Epstein-Barr virus, varicella zoster virus | • Consumption of toxins or poisons (e.g., puffer fish poisoning, lead, thallium, arsenic) |
| • Rabies virus, HIV | • Tick paralysis, Lyme disease |
| Transverse Myelitis | • Porphyria |
| • Mycoplasma pneumoniae | Neuromuscular Junction Disorders |
| • Herpes simplex virus, cytomegalovirus, Epstein-Barr virus, varicella zoster virus | • Myasthenia gravis |
| Spinal Cord Injury | • Lambert-Eaton myasthenic syndrome |
| • Acute spinal stenosis (e.g., disc prolapse, epidural abscess, or hematoma) | • Botulism |
| • Anterior spinal artery occlusion | Muscle Disorders |
| Neuromuscular Weakness Related to Critical Illness | • Acute myositis |
| • Critical illness neuropathy and myopathy | • Periodic paralysis |
| Parameter | Level | Units |
|---|---|---|
| Hemoglobin | 134.0 | g/L |
| Erythrocyte count | 4.5 | x1012/L |
| Platelet count | 205.0 | x109/L |
| Leukocyte count | 12.8 | x109/L |
| Urea | 6.68 | mmol/L |
| Creatinine | 124.8 | μmol/L |
| C-reactive protein (CRP) | 26.84 | mg/L |
| Procalcitonin (PCT) | 0.192 | ng/ml |
| Aspartate aminotransferase (AST) | 46.39 | U/L |
| Alanine aminotransferase (ALT) | 40.36 | U/L |
| Sodium (Na +) | 134.1 | mmol/L |
| Potassium (K+) | 4.9 | mmol/L |
| Chloride ion (Cl-) | 98.7 | mmol/L |
| Glucose (Glu) | 5.8 | mmol/L |
| N-Terminal Pro–B-Type Natriuretic Peptide (NT-proBNP) |
9516 | pg/mL |
2.7. Imaging Examinations
Duplex ultrasound of the lower extremity arteries was performed as the first imaging modality. Monophasic flow in the external iliac arteries and arteries below the groin was revealed. In addition, occlusion of the infrarenal part of the abdominal aorta and common iliac arteries was also revealed.
Computed tomographic angiography (CTA) of the aorta, its main branches, and the lower extremity arteries confirmed embolic occlusion of the infrarenal abdominal aorta and both common iliac arteries (Fig. 1). The abdominal aorta was completely occluded from the origin of the inferior mesenteric artery to its bifurcation, spanning a total length of 2.8 cm. The left common iliac artery was occluded from its origin to approximately 1.9 cm above its bifurcation, measuring 4.1 cm in total length. Similarly, the right common iliac artery was occluded from its origin to about 1 cm above its bifurcation, with a total length of 5.1 cm. Additionally, the CTA revealed the embolic source—an intracardiac thrombus adherent to the septum of the left atrium, as well as the apex and lateral wall of the left ventricle (LV)—which carried a high embolic potential (Fig. 2).
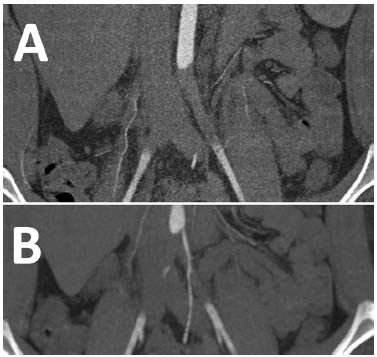
Computed tomographic angiography of the abdominal aorta. A: Coronal and B: Coronal postprocessing views demonstrating complete occlusion of the abdominal aorta and common iliac arteries.

Computed tomographic angiography. A: Picture (axial view) shows an intracardiac thrombus in the left atrium adhering to the interatrial septum and an intracardiac thrombus in the left ventricle adhering to the apex of the left ventricle (red arrows); B: Picture (axial view) shows an intracardiac thrombus in the left ventricle adhering to the lateral wall and apex of the left ventricle (red arrow).
2.8. Multidisciplinary Expert Consultation
The patient was evaluated by multidisciplinary experts in neurology, angiology, cardiology, radiology, and vascular surgery during the admission period and then by experts in angiology, cardiology, and cardiac surgery. Based on the patient's history mentioned above, he was preliminarily diagnosed with Guillain-Barré syndrome by paramedics, so a neurological examination was performed first in the emergency room.
The neurologist noted flaccid symmetrical weakness of the distal parts of the lower extremities and decreased deep tendon reflexes.
However, due to the presence of several concerning signs and symptoms, specifically intense pain in the lower extremities, rapid progression of neurological deficits within four hours of pain onset, and marked pallor and coldness of the lower limbs, the initial diagnosis was questioned and ultimately ruled out following a comprehensive neurological examination.
The patient was subsequently sent to a cardiovascular center for further differential diagnosis. Based on the patient's history, physical examination, and vascular ultrasound results, the prior preliminary diagnosis was reconsidered; according to Rutherford, bilateral acute limb ischemia of Grade IIB was the reason for the patient's complaints.
Since the limbs were at risk due to severe ischemia, revascularization was needed immediately, and a vascular surgeon was consulted.
In addition, a cardiologist was called, and echocardiography was performed before the surgical procedure. Severe systolic dysfunction of the left ventricle with an ejection fraction (EF)of 24%, dilatation of the left ventricle to 66 mm, and thrombus in the left ventricle were detected.
2.9. Final Diagnosis
The case was finally concluded to be a bilateral acute limb ischemia of Grade IIB according to Rutherford classification, caused by acute embolic aortic occlusion due to intracardiac thrombus formation after parvovirus B19 myocarditis (see diagnostic details below).
2.10. Treatment
Open surgical thrombo-embolectomy of the aorta and iliac arteries via both common femoral arteries was performed. Surgical revascularization was successful; however, immediately after the procedure, the patient developed ischemia-reperfusion injury complicated by compartment syndrome of the lower extremities. Thus, fasciotomy had to be performed. The histopathological study of material extracted from the aorta by a vascular surgeon confirmed the presence of thrombus and excluded myxoma.
2.11. Outcome and Follow-up
After surgical revascularization, the patient was transferred to the coronary unit for differential diagnosis of new-onset severe systolic dysfunction and dilatation of the left ventricle. Based on the main complaints, basic echocardiographic findings, and significant differences in territorial longitudinal strain between the endocardium and epicardium documented by echocardiography, myocarditis was suspected. Therefore, cardiac magnetic resonance imaging was performed.
Severe dilatation of the left ventricle to 73 mm, severe systolic dysfunction of the left ventricle with an ejection fraction of 14%, and edematous areas in the myocardium were documented. However, the diagnosis of myocarditis was not unambiguous due to artifacts caused by sinus tachycardia.
Subsequently, positron emission tomography of the heart was performed, documenting significantly increased metabolic activity in the myocardium, which was interpreted as myocarditis.
Finally, an endomyocardial biopsy was performed because the suspicion of myocarditis was still high. Standardized diagnostic criteria for histopathological analyses (Dallas criteria for myocarditis) were fulfilled. Quantitative polymerase chain reaction (PCR), reverse transcription (RT)-PCR, and direct sequencing were used to identify infectious agents, specifically parvovirus B19. Thus, the patient's viral myocarditis was finally confirmed.
Therapy upon discharge from the hospital included: warfarin according to the international normalized ratio (INR), furosemide 60 mg/day, eplerenone 50 mg/day, ivabradine 7.5 mg twice/day, carvedilol 12.5 mg twice/day, and trandolapril 0.5 mg/day.
The intracardiac thrombus completely resolved three months after anticoagulation was initiated. However, the EF of LV gradually worsened during follow-up despite adequate conservative treatment for heart failure, and fully symptomatic chronic heart failure developed.
Five years after the confirmed diagnosis of myocarditis, the patient underwent heart transplantation due to refractory heart failure. The arterial system of the lower extremities remained patent during this period.
3. DISCUSSION
In this article, we present a unique case of acute paraparesis in a young man who was initially diagnosed with Guillain-Barré syndrome. However, after a thorough differential diagnostic workup, the initial diagnosis was ruled out, and the paraparesis was attributed to severe ischemia caused by acute aortic occlusion from an embolus that originated in the left ventricle as a consequence of myocarditis.
Left ventricular thrombus represents a potentially life-threatening condition due to the significant risk of stroke and systemic thromboembolism. The incidence of left ventricular thrombus after ST-segment elevation myocardial infarction (STEMI) varies widely among different reports, ranging from 4% to 39% [4, 5].
Furthermore, the incidence of left ventricular thrombus in patients with dilated (nonischemic) cardiomyopathy may be between 2% and 36% [4, 6, 7]. In the past, the presence of a left ventricular thrombus has been associated with a risk of up to 22% of embolization [4, 7] and a 37% risk of major adverse cardiovascular events [6].
A widely accepted hypothesis posits that the pathogenesis of left ventricular thrombus is a result of the coexistence of 3 factors: 1) stasis due to reduced ventricular function, 2) endocardial injury, and 3) inflammation/hypercoagulability.
Thus, the factors mentioned above can contribute to the formation of left ventricular thrombus under different cardiac conditions.
Acute aortic occlusion (AAO) with bilateral lower limb ischemia is an immediately life-threatening condition with an incidence of 3.8 per million person-years [8-10]. It can be caused by large saddle emboli from the heart; by thrombosis of an atherosclerotic or aneurysmal aorta; less commonly, it can be secondary to thrombophilia or low cardiac output; or by acute occlusion of a previously inserted graft or stent graft.
The management of AOO remains challenging even in the modern era, and delay is associated with poor outcomes owing to severe ischemia, resulting in devastating complications of ischemia-reperfusion injury. Thus, in patients with AAO, urgent revascularization is mandatory [8].
The 30-day mortality rate after surgical revascularization tends to improve; however, it still remains severely high at 15.5%, even in the 21st century [10].
There is no consensus regarding the best method of revascularization. However, thrombo-embolectomy, catheter-directed thrombolytic therapy, axillobifemoral bypass, and aorto-bi-iliac or -bifemoral bypass are potential options for revascularization. Professionals should consider etiology, comorbidities, resources, and experience when making decisions, which should be based on standard vascular surgical principles [8].
CONCLUSION
Both pathologies, i.e., myocarditis with left ventricular thrombus and acute aortic occlusion, are life-threatening pathologies that require skilled and experienced physicians for diagnosis, clinical evaluation, and treatment. Thorough initial physical examination plays a crucial role in correct differential diagnosis and decision-making, which would definitely reduce the occurrence of severe consequences of ischemia in our patient.
Myocarditis is one of the risk factors causing intracardiac thrombus formation and consequently increases the risk of peripheral embolization. The combination of neurological defect due to embolization resulting in severe peripheral ischemia, and fever associated with antecedent infectious illness leading to myocarditis can be misinterpreted and misdiagnosed as GBS. However, rapid onset and progression of neurological defects, pulseless palpation of peripheral arteries, and pale, cold lower extremities are strong signs that exclude GBS.
AUTHORS' CONTRIBUTIONS
The authors confirm their contribution to the paper as follows: study conception and design: MR; analysis and interpretation of results: MK; Writing the Paper: MH. All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| GBS | = Guillain-Barré syndrome |
| CTA | = Computed tomographic angiography |
| LV | = Left ventricle |
| EF | = Ejection fraction |
| PCR | = Polymerase chain reaction |
| RT | = Reverse transcription |
| INR | = International normalized ratio |
| STEMI | = ST-segment elevation myocardial infarction |
| AAO | = Acute aortic occlusion |
ETHICAL STATMENT
The need for ethical approval was waived. Consent from the patient is deemed to be enough.
CONSENT FOR PUBLICATION
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
AVAILABILITY OF DATA AND MATERIALS
The data and supportive information are available within the article.
ACKNOWLEDGEMENTS
Declared none.


